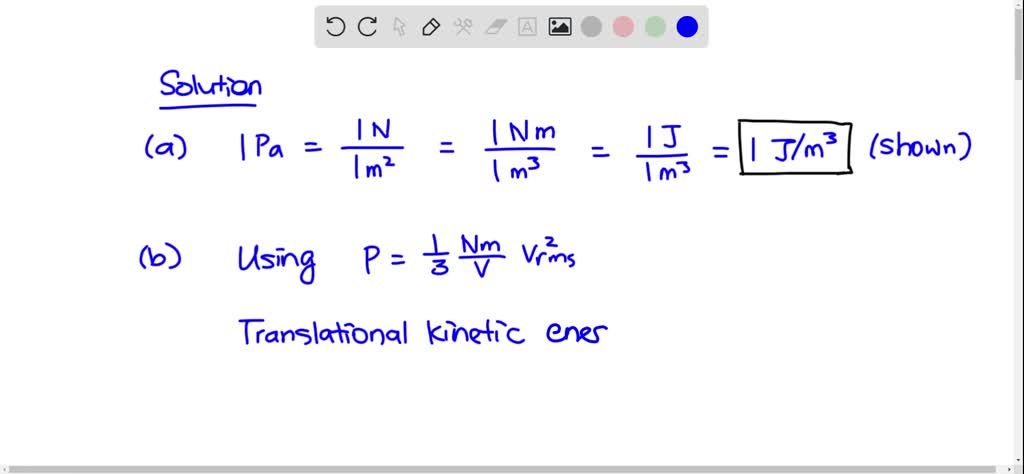
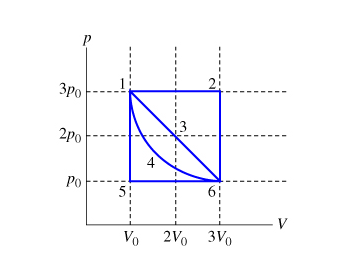
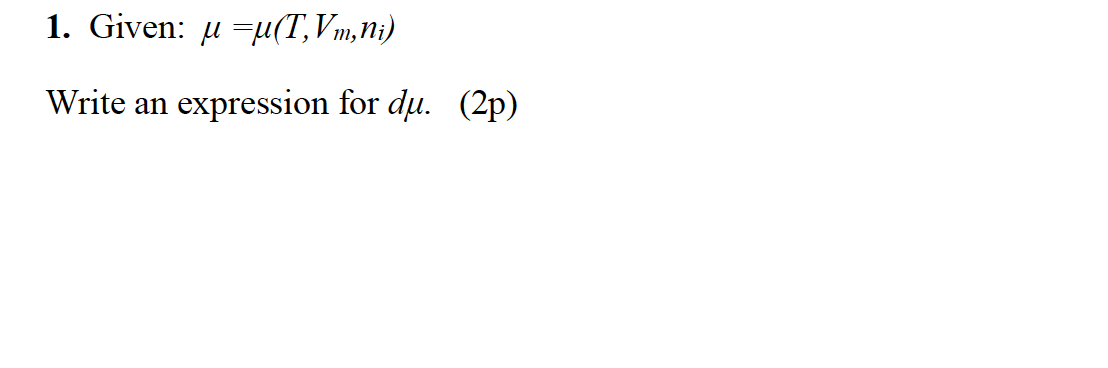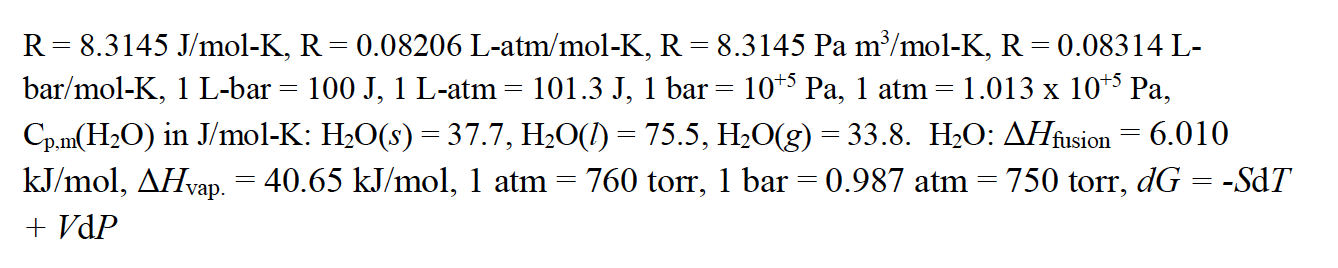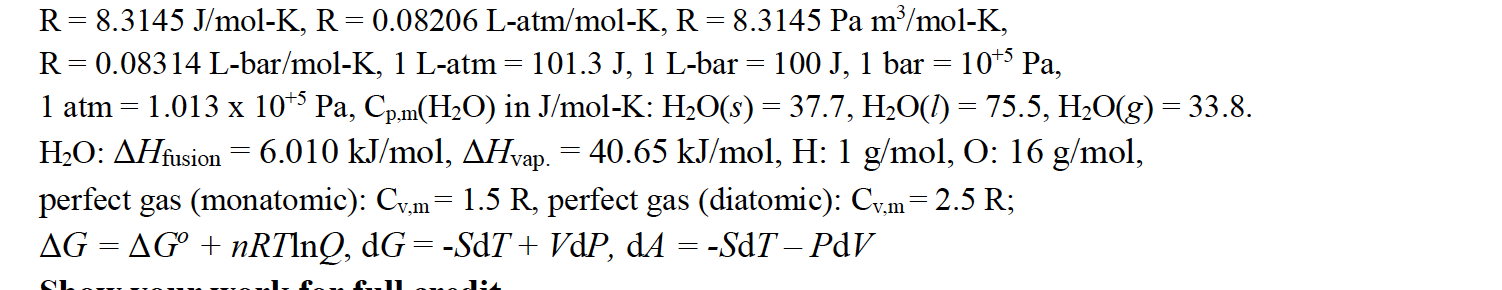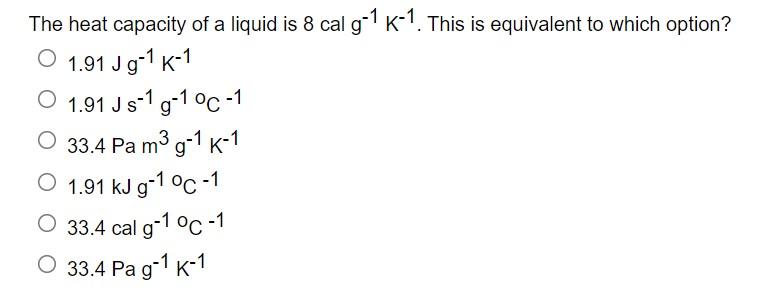SOLVED: A sample of an ideal gas is initially at a volume of 3.5 L. The gas expands to a volume of 7.0 m3 when 2 J of heat is applied to
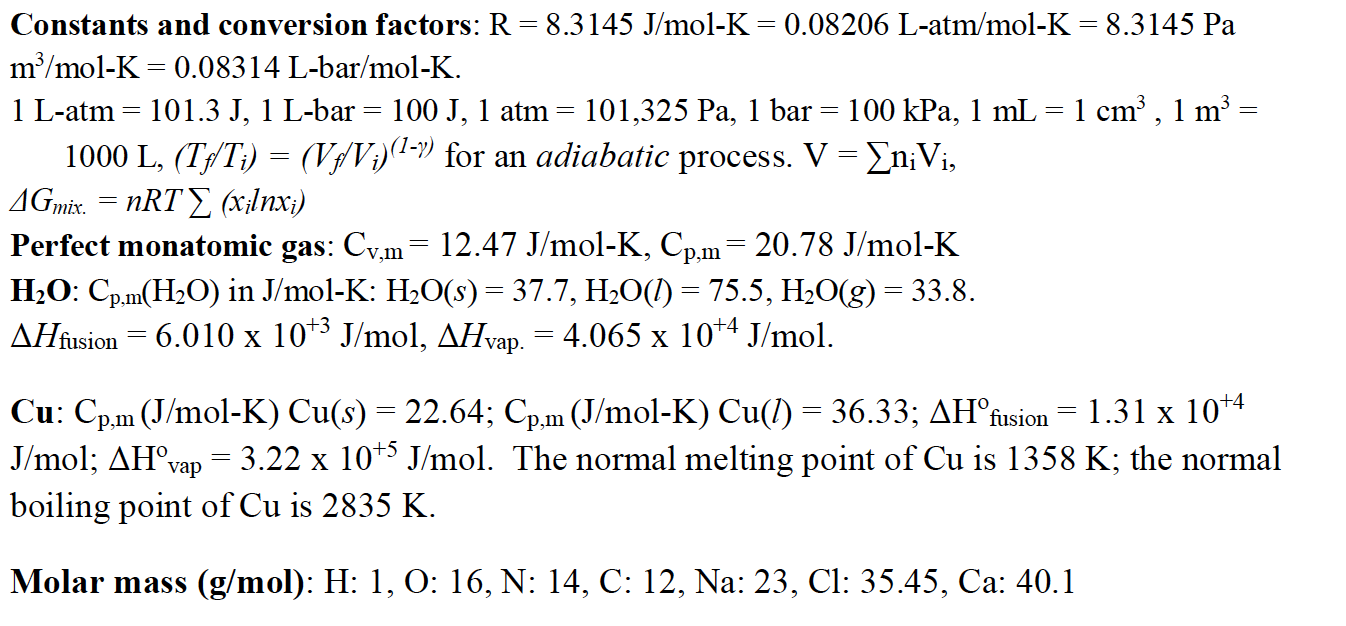
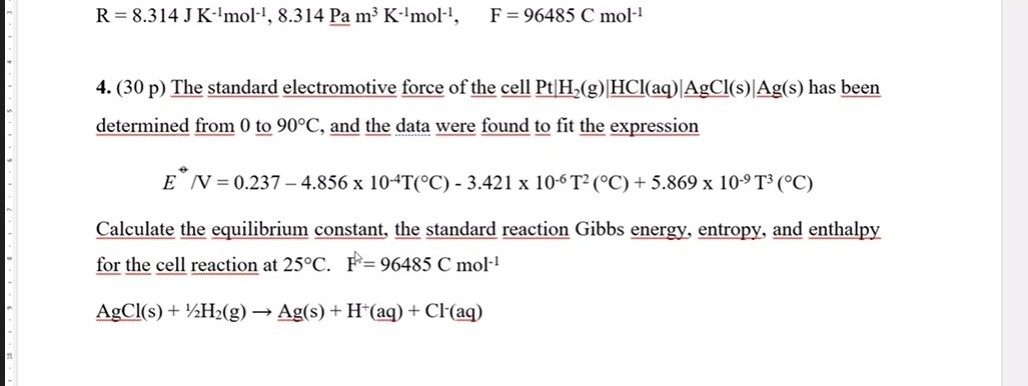
![SOLVED: Fundamental constants and conversion factors: NA 6.022 *1023 mol-1 k (or kB) 71381*10-23] K-1 R=kNA 8.314 ] K-l mol-! = 8.314 Pa m3 mol-! E K-l 1.602 x10-19 C NAe = SOLVED: Fundamental constants and conversion factors: NA 6.022 *1023 mol-1 k (or kB) 71381*10-23] K-1 R=kNA 8.314 ] K-l mol-! = 8.314 Pa m3 mol-! E K-l 1.602 x10-19 C NAe =](https://cdn.numerade.com/ask_images/de3c0ad446614f81852d19db635e597b.jpg)
SOLVED: Fundamental constants and conversion factors: NA 6.022 *1023 mol-1 k (or kB) 71381*10-23] K-1 R=kNA 8.314 ] K-l mol-! = 8.314 Pa m3 mol-! E K-l 1.602 x10-19 C NAe =

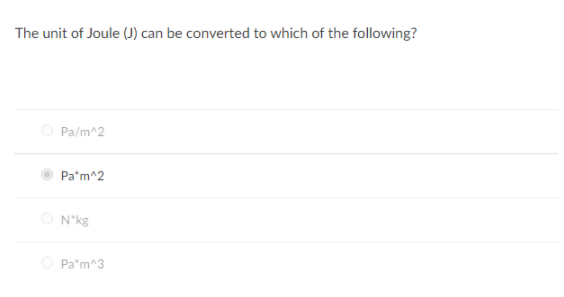
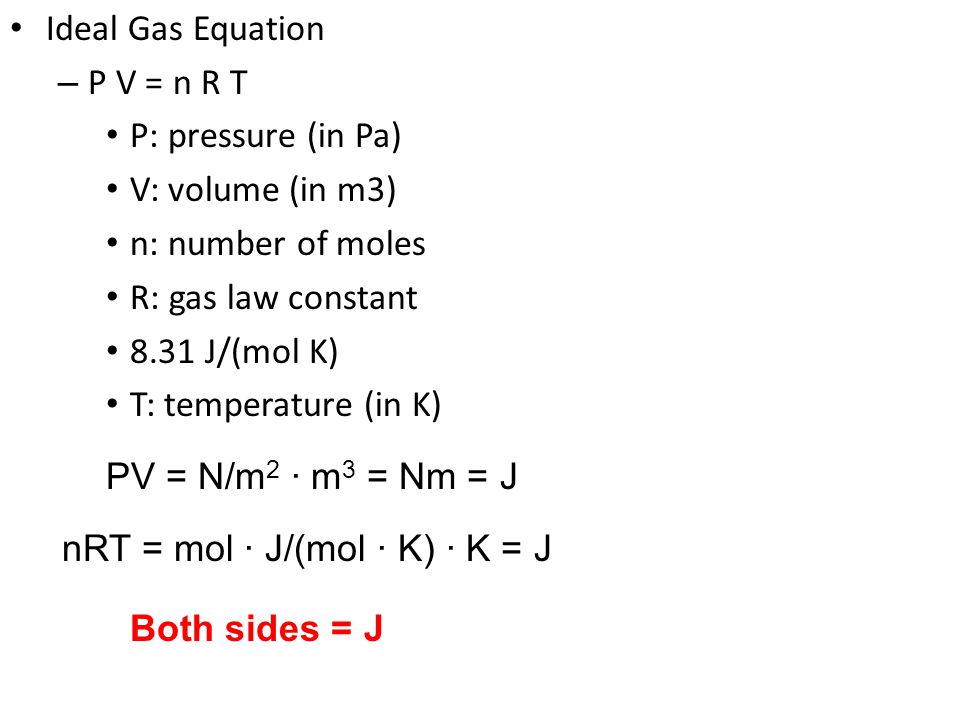
SOLVED: There are four additive terms in an equation, and their units are given below. Which one is not consistent with this equation? (a) J (b) W/m (c) kg·m2/s2 (d) Pa·m3 (e)
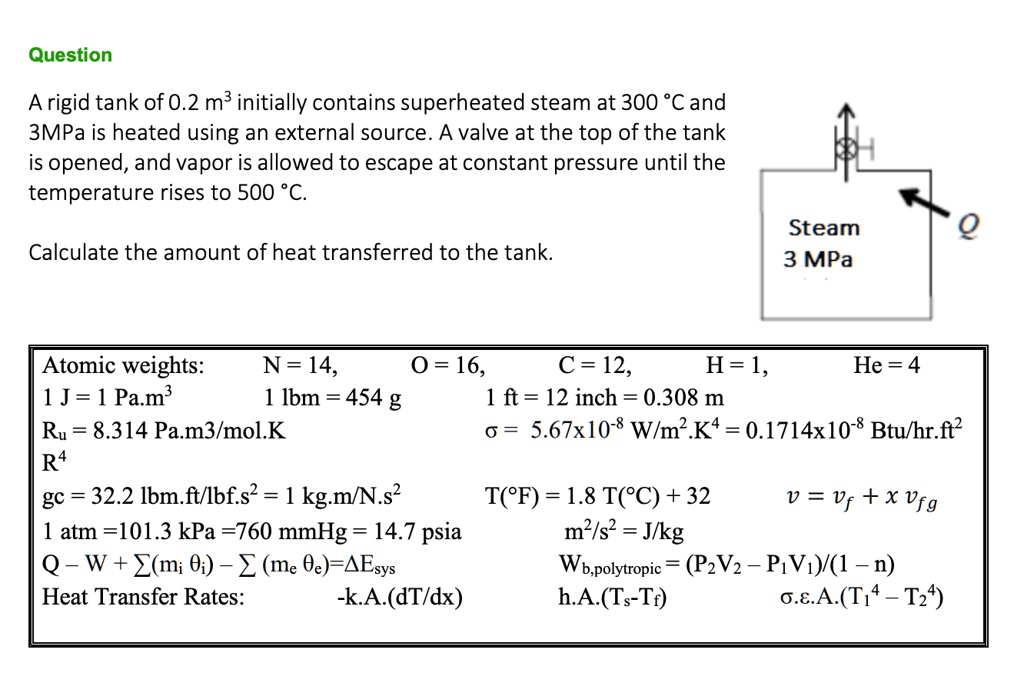
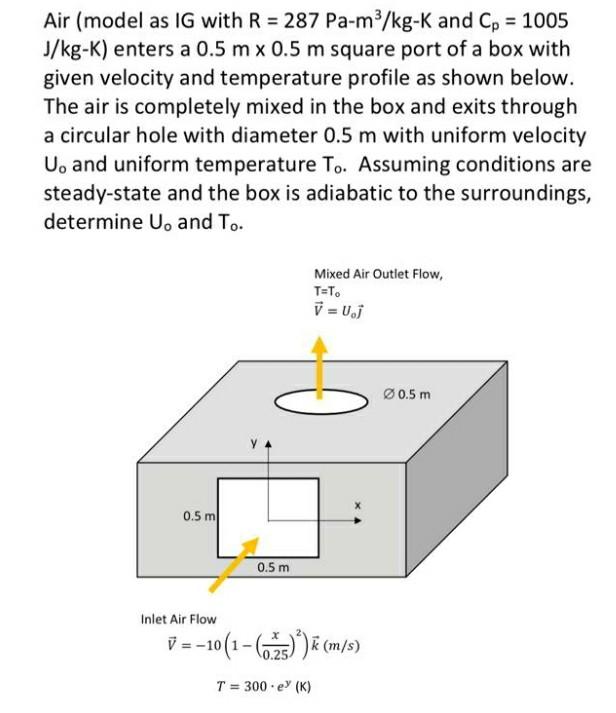
SOLVED: Question A rigid tank of 0.2 m3 initially contains superheated steam at 300 'C and 3MPa is heated using an external source. A valve at the top of the tank is

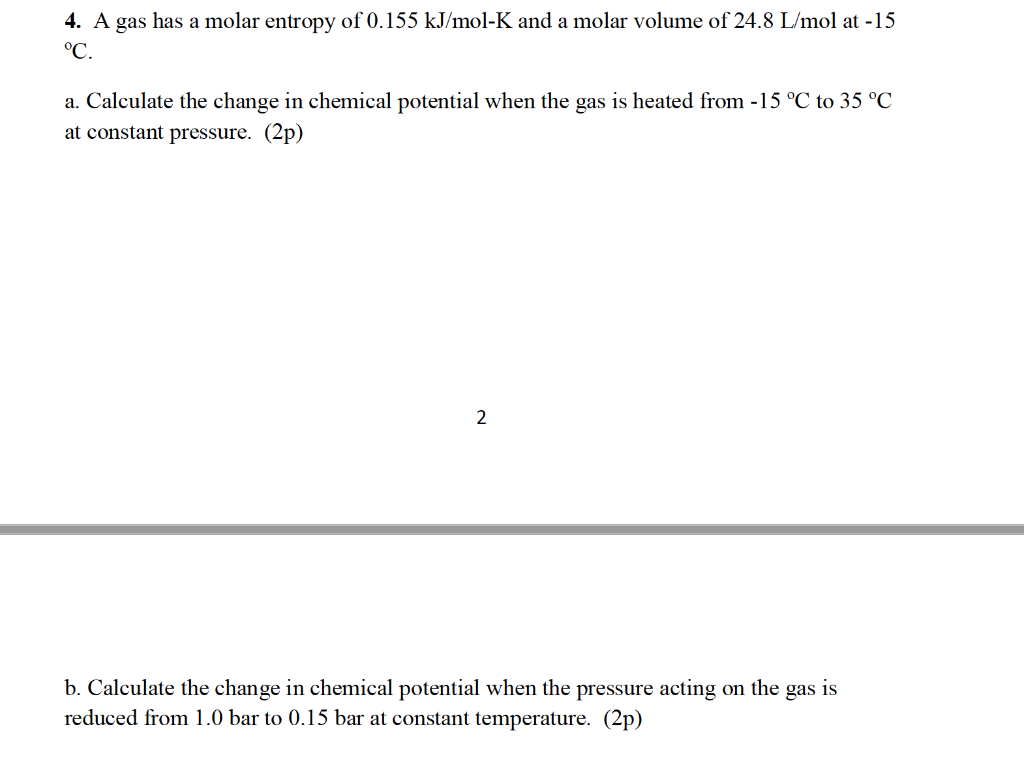
Notes for CHEM1022, Chemistry for Pharmacy and Dentistry | CHEM1222 - Chemistry for Pharmacy and Dentistry - UQ | Thinkswap